MediView receives FDA 510(okay) clearance for its XR90 platform
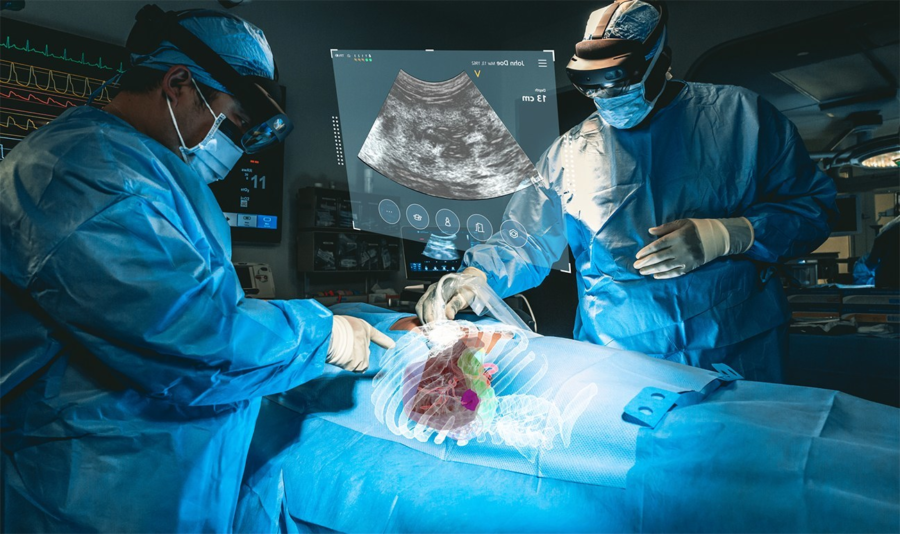
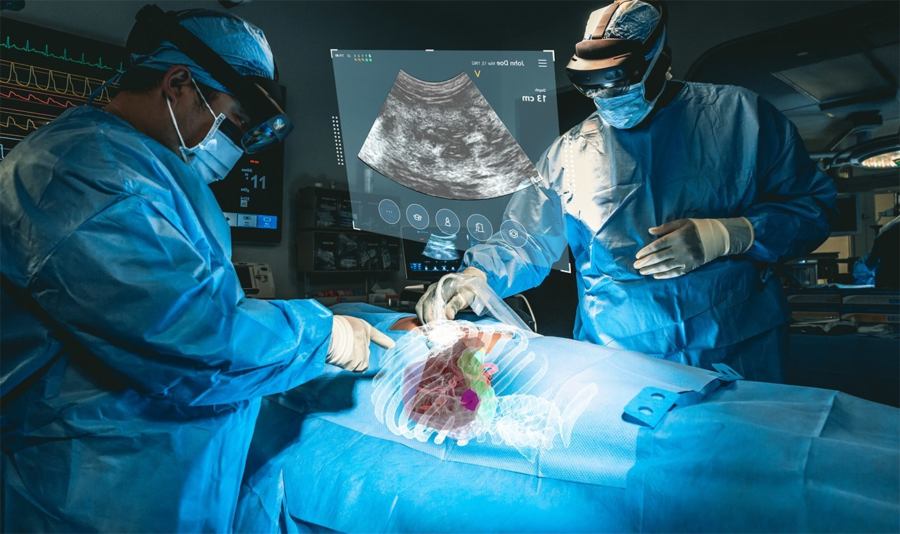
July 20, 2023 – MediView XR, Inc., a medical augmented actuality (AR) med-tech firm, has right this moment introduced that it has obtained 510(okay) clearance from the U.S. Meals and Drug Administration (FDA) for its XR90 augmented reality-based surgical visualization and navigation platform.
MediView makes use of AR to deal with among the limitations of present medical imaging applied sciences related to flat panel displays, which restrict practitioners to 2D imaging and require them to look away from a affected person’s procedural website. The corporate’s XR90 system is meant for use adjunctively to plain of care imaging for minimally invasive ultrasound and CT guided needle-based procedures for comfortable tissue and bone.
The system initiatives 3D digital fashions of a affected person’s personal anatomy based mostly on their CT imaging and combines that CT with reside ultrasound to carry out minimally invasive procedures, similar to biopsies and tumor ablations (using warmth or chilly to kill cancerous tumors).
XR90’s augmented actuality capabilities embrace a Holographic Mild Ray that tracks and shows the trail of the doctor’s instrument, CT-based 3D holographic anatomy show, and reside ultrasound that’s projected and displayed anatomically into the affected person because the clinician scans, much like a flashlight beam.
By means of Microsoft’s HoloLens 2 AR headset, clinicians are capable of visualize the affected person’s ultrasound, in addition to shows of different procedural info to facilitate their workflow. MediView acknowledged that the XR90 overcomes the constraints of two-dimensional imaging by offering physicians with 3D “X-Ray imaginative and prescient” throughout procedures – the power to visualise a affected person’s complete inner anatomy in 3D beneath their pores and skin, together with bone, tissue, organs and vasculature.
“XR90 expands the MediView portfolio of options out there to practitioners as they appear to simplify, democratize, and inform care supply with the final word objective of enhancing and increasing entry to the most effective care,” stated Mina Fahim, President and CEO of MediView.
MediView’s expertise additionally permits clinicians at distant areas to collaborate in real-time with shared visualization, communication, and the power to supply steerage throughout procedures for collaborative affected person care. These options can present elevated help for understaffed amenities, rural or underserved populations and a definite capability to restrict caregiver and affected person publicity to COVID-19 whereas sustaining care ranges, in keeping with MediView.
Commenting on the announcement, Adam Cargill, Director of High quality, Regulatory and Scientific Affairs at MediView, stated: “This isn’t solely the primary 510(okay) clearance for MediView, however it’s the first 510(okay) clearance for an augmented actuality system using reside imaging mixed with 3D XR visualization for pre- and intra-operative indications to be used, which units the stage for additional developments in augmented actuality within the healthcare house.”
MediView recently announced that it secured a USD $15 million strategic funding spherical to develop its AR surgical navigation platform. To study extra about MediView’s augmented actuality options for medical imaging, please go to the corporate’s website.
Picture credit score: MediView
Sam Sprigg
Sam is the Founder and Managing Editor of Auganix. With a background in analysis and report writing, he has been protecting XR business information for the previous 5 years.